AliveCor
Founded Year
2011Stage
Series F | AliveTotal Raised
$134.33MAbout AliveCor
AliveCor focuses on the creation of FDA-cleared machine learning techniques to enable proactive heart care. The FDA-cleared KardiaMobile is a clinically validated mobile EKG solution used by people for accurate EKG recordings. KardiaMobile, and KardiaBand, when paired with the Kardia app provide instant analysis for detecting atrial fibrillation (AF) and normal sinus rhythm in an EKG. The firm serves clients operating in the healthcare sector. It was formerly known as AliveUSA. It was founded in 2011 and is based in Mountain View, California.
Missing: AliveCor's Product Demo & Case Studies
Promote your product offering to tech buyers.
Reach 1000s of buyers who use CB Insights to identify vendors, demo products, and make purchasing decisions.
ESPs containing AliveCor
The ESP matrix leverages data and analyst insight to identify and rank leading companies in a given technology landscape.
The audio biomarkers — lung health market is a growing industry that utilizes audio technology to detect and monitor lung diseases such as asthma, chronic obstructive pulmonary disease (COPD), and pneumonia. This technology can analyze the sound of a patient's breathing and coughing to identify potential issues before they become more serious. The use of audio biomarkers can also provide a non-inv…
AliveCor named as Leader among 15 other companies, including Innovaccer, TytoCare, and Sensely.
Missing: AliveCor's Product & Differentiators
Don’t let your products get skipped. Buyers use our vendor rankings to shortlist companies and drive requests for proposals (RFPs).
Research containing AliveCor
Get data-driven expert analysis from the CB Insights Intelligence Unit.
CB Insights Intelligence Analysts have mentioned AliveCor in 12 CB Insights research briefs, most recently on Jan 27, 2023.
Oct 14, 2022
What is Best Buy doing in healthcare?
Expert Collections containing AliveCor
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
AliveCor is included in 14 Expert Collections, including Clinical Trials Tech.
Clinical Trials Tech
341 items
Companies developing products and services to streamline drug R&D, from drug discovery, pre-clinical testing, and clinical trials.
Tech IPO Pipeline
286 items
Artificial Intelligence
10,624 items
This collection includes startups selling AI SaaS, using AI algorithms to develop their core products, and those developing hardware to support AI workloads.
Value-Based Care & Population Health
1,066 items
The VBC & Population Health collection includes companies that enable and deliver care models that address the health needs for defining populations along the continuum of care, including in the community setting, through participation, engagement, and targeted interventions.
Medical Devices
12,161 items
Companies developing medical devices (per the IMDRF's definition of "medical device"). Includes software, lab-developed tests (LDTs), and combination products. *Columns updated as regularly as possible.
Digital Hospital
298 items
Startups recreating how healthcare is delivered
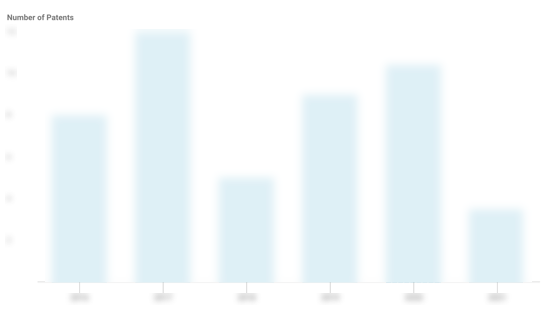
AliveCor Patents
AliveCor has filed 75 patents.
The 3 most popular patent topics include:
- Cardiac arrhythmia
- Cardiac electrophysiology
- Cardiology
Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
3/7/2018 | 1/24/2023 | Cardiac arrhythmia, Cardiac electrophysiology, Cardiology, Electrophysiology, Cardiac procedures | Grant |
Application Date | 3/7/2018 |
|---|---|
Grant Date | 1/24/2023 |
Title | |
Related Topics | Cardiac arrhythmia, Cardiac electrophysiology, Cardiology, Electrophysiology, Cardiac procedures |
Status | Grant |
Latest AliveCor News
May 3, 2023
News provided by Share this article KardiaMobile Card recognized for its contributions to digital health MOUNTAIN VIEW, Calif., May 3, 2023 /PRNewswire/ -- AliveCor , the leading innovator in FDA-cleared personal electrocardiogram (ECG) technology, today announced that KardiaMobile Card , the world's thinnest and lightest personal ECG, has been honored on Fast Company's 2023 World Changing Ideas list in the Health category and awarded the 2023 MedTech Breakthrough Award for Best New Technology Solution in the Cardiology category. KardiaMobile Card is the world's first credit-card-sized personal ECG device. With the ability to detect six of the most common arrhythmias in just 30 seconds, KardiaMobile Card provides patients with the critical data they need to take charge of their heart health at home or on the go. "At AliveCor, we're relentless in our pursuit to improve personal cardiac monitoring and are honored to be recognized by Fast Company and MedTech Breakthrough for our efforts in bringing our advanced, clinically validated AI directly into patients' wallets and back pockets," said Jessica Weinstein, Senior Vice President, Global Marketing, AliveCor. "KardiaMobile Card represents the future of heart health, and we're excited to be at the forefront of improving cardiovascular care delivery by making essential data more accessible." Fast Company's annual list of World Changing Ideas spotlights individuals and organizations that are solving today's biggest challenges. The 2023 honorees reflect some of the most transformative ideas across all industries. The MedTech Breakthrough Awards, organized by MedTech Breakthrough, an independent market intelligence organization, honors the platforms, devices and people who are making a difference in the healthcare industry. About AliveCor AliveCor, Inc. is transforming cardiological care using deep learning. The FDA-cleared KardiaMobile device is the most clinically-validated personal ECG solution in the world. KardiaMobile 6L provides instant detection of Atrial Fibrillation, Bradycardia, Tachycardia, Sinus Rhythm with Supraventricular Ectopy, Sinus Rhythm with Premature Ventricular Contractions, Sinus Rhythm with Wide QRS, and Normal Sinus Rhythm in an ECG. Kardia is the first AI-enabled platform to aid patients and clinicians in the efficient detection of atrial fibrillation, the most common arrhythmia and one associated with a highly-elevated risk of stroke. AliveCor's enterprise platform allows third-party providers to manage their patients' and customers' heart conditions simply using state-of-the-art tools that provide easy front-end and back-end integration to AliveCor technologies. AliveCor protects its customers with stringent data security and compliance practices , achieving ISO 27001 Certification, SOC 2 Type 2 Certification and HIPAA compliance attestation. AliveCor is a privately-held company headquartered in Mountain View, Calif. "Consumer" or "Personal" ECGs are ECG devices available for direct sale to consumers. For more information, visit alivecor.com . SOURCE AliveCor, Inc.
AliveCor Frequently Asked Questions (FAQ)
When was AliveCor founded?
AliveCor was founded in 2011.
Where is AliveCor's headquarters?
AliveCor's headquarters is located at 444 Castro Street, Mountain View.
What is AliveCor's latest funding round?
AliveCor's latest funding round is Series F.
How much did AliveCor raise?
AliveCor raised a total of $134.33M.
Who are the investors of AliveCor?
Investors of AliveCor include Qualcomm Ventures, Khosla Ventures, BOLD Capital Partners, WP Global Partners, GE Healthcare and 9 more.
Who are AliveCor's competitors?
Competitors of AliveCor include Healthy.io, Powerful Medical, Eko, Wellysis, Heartbeat Health and 11 more.
Compare AliveCor to Competitors

Happitech develops a certified heart rhythm software development kit for android and i-phone operating systems. The company offers algorithms that allow medical-grade vital signs monitoring directly through a smartphone. The company was founded in 2015 and is based in Amsterdam, Netherlands.
Heart Care helps users have healthier routines and cultivates the habit of constant health monitoring. Heart Care is not a clinical tool, but a health and wellness application. The app is a simple and intuitive design, where users can easily find all the tools they need to increase health and well-being.

Eko creates digital health solutions. Its solutions include artificial intelligence (AI) enabled stethoscopes, patient and provider software, and AI-powered analytics that enable doctors, nurses, researchers, and entire health systems to change care for the heart. It serves clients in the healthcare sector. The firm was formerly known as Eko Health. It was founded in 2013 and is based in Oakland, California.

Steth IO offers a telemedicine platform. It develops medical devices to increase the accuracy of essential medical practices. The company offers products that include a smartphone stethoscope for visualizing and hearing heart sounds. It was founded in 2015 and is based in Bothell, Washington.

Vatic Health provides healthcare solutions. The company develops rapid test kits. It was formerly known as my110. The company was founded in 2019 and is based in London, United Kingdom.
Ventricle Health offers a digital platform that allows expert cardiologists to monitor patients' heart health remotely. The company's platform provides timely telehealth visits with an assigned board-certified cardiologist, one-on-one sessions with an assigned health coach to make healthy lifestyle changes, and a pharmacy specialist to help with medications. It was founded in 2021 and is based in Greensboro, North Carolina.
Discover the right solution for your team
The CB Insights tech market intelligence platform analyzes millions of data points on vendors, products, partnerships, and patents to help your team find their next technology solution.