Denali Therapeutics
Founded Year
2015Stage
IPO | IPOTotal Raised
$347MDate of IPO
12/8/2017Market Cap
3.62BStock Price
26.52About Denali Therapeutics
Denali Therapeutics (NASDAQ: DNLI) is a biotechnology company focused on the discovery and development of therapies for patients with neurodegenerative diseases, including Alzheimer's disease, Parkinson's disease, ALS, and others. It pursues treatments by assessing genetically validated targets, engineering delivery, and guiding development through biomarkers that demonstrate target and pathway engagement. The company was founded in 2015 and is based in South San Francisco, California.
Missing: Denali Therapeutics's Product Demo & Case Studies
Promote your product offering to tech buyers.
Reach 1000s of buyers who use CB Insights to identify vendors, demo products, and make purchasing decisions.
Missing: Denali Therapeutics's Product & Differentiators
Don’t let your products get skipped. Buyers use our vendor rankings to shortlist companies and drive requests for proposals (RFPs).
Expert Collections containing Denali Therapeutics
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
Denali Therapeutics is included in 1 Expert Collection, including Biopharma Tech.
Biopharma Tech
1,568 items
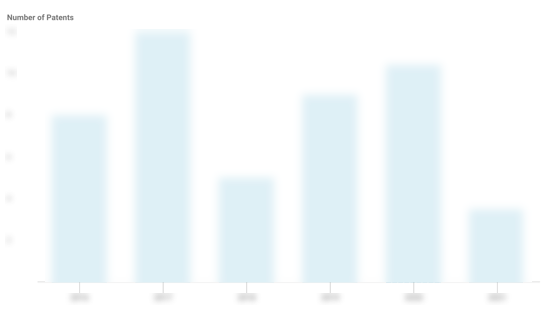
Denali Therapeutics Patents
Denali Therapeutics has filed 75 patents.
The 3 most popular patent topics include:
- Neurological disorders
- Rare diseases
- Clusters of differentiation
Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
10/19/2018 | 3/28/2023 | Clusters of differentiation, Transcription factors, Molecular biology, Proteins, Genetics | Grant |
Application Date | 10/19/2018 |
|---|---|
Grant Date | 3/28/2023 |
Title | |
Related Topics | Clusters of differentiation, Transcription factors, Molecular biology, Proteins, Genetics |
Status | Grant |
Latest Denali Therapeutics News
Apr 21, 2023
nature reviews clinical oncology Abstract Antibody–drug conjugates (ADCs), a class of targeted cancer therapeutics combining monoclonal antibodies with a cytotoxic payload via a chemical linker, have already been approved for the treatment of several cancer types, with extensive clinical development of novel constructs ongoing. Primary and secondary brain tumours are associated with high mortality and morbidity, necessitating novel treatment approaches. Pharmacotherapy of brain tumours can be limited by restricted drug delivery across the blood–brain or blood–tumour barrier, although data from phase II studies of the HER2-targeted ADC trastuzumab deruxtecan indicate clinically relevant intracranial activity in patients with brain metastases from HER2+ breast cancer. However, depatuxizumab mafodotin, an ADC targeting wild-type EGFR and EGFR variant III, did not provide a definitive overall survival benefit in patients with newly diagnosed or recurrent EGFR-amplified glioblastoma in phase II and III trials, despite objective radiological responses in some patients. In this Review, we summarize the available data on the central nervous system activity of ADCs from trials involving patients with primary and secondary brain tumours and discuss their clinical implications. Furthermore, we explore pharmacological determinants of intracranial activity and discuss the optimal design of clinical trials to facilitate development of ADCs for the treatment of gliomas and brain metastases. Key points Antibody–drug conjugates (ADCs) have shown promising clinical activity across several different solid tumour entities; however, data on the intracranial efficacy of these agents are scarce, especially in patients with active brain metastases. Data from phase II trials suggest that the HER2-targeted ADC trastuzumab deruxtecan has substantial central nervous system activity in patients with breast cancer brain metastases. By contrast, the EGFR-targeted ADC depatuxizumab mafodotin failed to produce an overall survival benefit in patients with glioblastoma. Pharmacological determinants of the intracranial activity of ADCs include the linker design, payload used, and the drug-to-antibody ratio and its heterogeneity in the ADC formulation. Dedicated, adequately powered clinical trials including rational end points of intracranial response are warranted to evaluate the efficacy of ADCs in patients with primary and secondary brain tumours. Access options Get Nature+, our best-value online-access subscription $29.99 per month $189.00 per year Get just this article for as long as you need it $39.95 Additional access options: References Ehrlich, P. The Harben Lectures, 1907. Experimental researches on specific therapeutics. J. R. Inst. Public Health 15, 321–340 (1907). Cardillo, T. M., Govindan, S. V., Sharkey, R. M., Trisal, P. & Goldenberg, D. M. Humanized anti-Trop-2 IgG-SN-38 conjugate for effective treatment of diverse epithelial cancers: preclinical studies in human cancer xenograft models and monkeys. Clin. Cancer Res. 17, 3157–3169 (2011). Diéras, V. et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 18, 732–742 (2017). ASCO Post. Second-line T-DXd improves progression-free survival across HER2-positive metastatic breast cancer subgroups. ASCO Post https://ascopost.com/issues/march-10-2022-supplement-conference-highlights-sabcs-2021/second-line-t-dxd-improves-progression-free-survival-across-her2-positive-metastatic-breast-cancer-subgroups/ (2022). Tarantino, P., Curigliano, G. & Tolaney, S. M. Navigating the HER2-low paradigm in breast oncology: new standards, future horizons. Cancer Discov. 12, 2026–2030 (2022). Pandey, R., Gruslova, A., Chiou, J., Brenner, A. J. & Tiziani, S. Stable isotope dilution LC-HRMS assay to determine free SN-38, total SN-38, and SN-38G in a tumor xenograft model after intravenous administration of antibody–drug conjugate (sacituzumab govitecan). Anal. Chem. 92, 1260–1267 (2020). Cleary, J. M. et al. A phase 1 study evaluating safety and pharmacokinetics of losatuxizumab vedotin (ABBV-221), an anti-EGFR antibody-drug conjugate carrying monomethyl auristatin E, in patients with solid tumors likely to overexpress EGFR. Invest. N. Drugs 38, 1483–1494 (2020). Coomans, M. et al. The added value of health-related quality of life as a prognostic indicator of overall survival and progression-free survival in glioma patients: a meta-analysis based on individual patient data from randomised controlled trials. Eur. J. Cancer 116, 190–198 (2019). Acknowledgements M.J.M, A.S.B and M.P. gratefully acknowledge financial support from the Austrian Federal Ministry for Digital and Economic Affairs, the Austrian National Foundation for Research, Technology and Development, and the Christian Doppler Research Association. The work of A.B.L. is supported in part, outside the submitted work, by The William Rhodes and Louise Tilzer-Rhodes Center for Glioblastoma at New York-Presbyterian Hospital, The Michael Weiner Glioblastoma Research Into Treatment Fund, and US National Institutes of Health (NIH)/National Cancer Institute (NCI) grants P30CA013696 and UG1CA189960. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH/NCI. Author information Maximilian J. Mair, Rupert Bartsch, Anna S. Berghoff & Matthias Preusser Christian Doppler Laboratory for Personalized Immunotherapy, Medical University of Vienna, Vienna, Austria Maximilian J. Mair, Anna S. Berghoff & Matthias Preusser Department of Neurosurgery, Clinical Neuroscience Center, University Hospital and University of Zurich, Zurich, Switzerland Emilie Le Rhun Emilie Le Rhun & Michael Weller Division of Hematology/Oncology, Department of Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA Priscilla K. Brastianos Priscilla K. Brastianos Javier Cortes Javier Cortes Javier Cortes Cancer Therapies and Biology Group, Centre of Research Excellence in Brain Tumours, Olivia Newton-John Cancer Wellness and Research Centre, Austin Hospital, Heidelberg, VIC, Australia Hui K. Gan Hui K. Gan Hui K. Gan Nancy U. Lin Division of Neuro-Oncology, Department of Neurology, Herbert Irving Comprehensive Cancer Center, Columbia University Vagelos College of Physicians and Surgeons and New York-Presbyterian Hospital, New York, NY, USA Andrew B. Lassman Patrick Y. Wen Patrick Y. Wen Martin van den Bent Rupert Bartsch Emilie Le Rhun Anna S. Berghoff Priscilla K. Brastianos Javier Cortes Hui K. Gan Nancy U. Lin Andrew B. Lassman Patrick Y. Wen Michael Weller Martin van den Bent Matthias Preusser Contributions M.J.M. and M.P. wrote the initial draft of the manuscript. All authors provided substantial intellectual input and reviewed and/or edited the manuscript prior to submission. Corresponding author Competing interests M.J.M. has received travel support from Pierre Fabre. E.LeR. has received grant/research support from Bristol Myers Squibb; and honoraria for lectures or advisory board participation from Adastra, Bayer, Janssen, Leo Pharma, Pierre Fabre and Seattle Genetics. A.S.B. has received research support from Daiichi Sankyo and Roche; honoraria for lectures, consultation or advisory board participation from Bristol Myers Squibb, Daiichi Sankyo, Merck and Roche; and travel support from AbbVie, Amgen and Roche. P.K.B. has consulted for Advice Connect Inspire, Angiochem, Axiom, Dantari, ElevateBio, Genentech/Roche, Lilly, MPM Capital, Pfizer, SK Life Sciences and Tesaro; has received institutional grant/research support (to Massachusetts General Hospital) from Bristol Myers Squibb, Lilly, Merck and Mirati; and has received honoraria from Genentech/Roche, Lilly, Merck and Pfizer (all outside the scope of this work). N.U.L. has received non-financial support from Seagen for manuscript preparation during the conduct of research studies; grants from AstraZeneca, Genentech, Merck, Olema Pharmaceuticals, Pfizer, Seagen and Zion Pharmaceuticals outside the submitted work; personal fees from Affinia Therapeutics, Aleta BioPharma, AstraZeneca, Daiichi Sankyo, Denali Therapeutics, Olema Pharmaceuticals, Pfizer, Prelude Therapeutics, Puma, Seagen and Voyager Therapeutics outside the submitted work; consulting fees from Affinia Therapeutics, Aleta BioTherapeutics, Artera, AstraZeneca, Daiichi Sankyo, Denali Therapeutics, Olema Oncology, Pfizer, Prelude Therapeutics, Seagen and Voyager Therapeutics; and royalties from UpToDate outside the submitted work. A.B.L. has received within the past 5 years travel, publication, other in-kind support, honoraria for consulting or advisory board participation and institutional research funding from Abbott and/or AbbVie, and in the past year has received travel, publication or other in-kind support from Chimerix, Karyopharm, Norwest Biotherapeutics, Pfizer, QED and VBI Vaccines; honoraria for consulting or advisory board participation from Affinia Therapeutics, Bioclinica (as an expert, blinded, independent reviewer of de-identified clinical data for a Bristol Myers Squibb-sponsored trial), Chimerix, Clinical Education Alliance, Leal Therapeutics, Novocure, Orbus and Sapience; and institutional research funding from AbbVie, Aeterna Zentaris, Bayer, Beigene, Bristol Myers Squibb, Cadmon, Chimerix, DelMar, Genentech/Roche, Kadmon, Karyopharm, Kazia, Keryx, Millennium, NextSource, Orbus, Pfizer, QED, Semus, Servier and VBI Vaccines. P.Y.W. has received honoraria for consulting or advisory board participation from AstraZeneca, Bayer, Black Diamond Therapeutics, Cellularity, Chimerix, Day One Biopharmaceuticals, Kazia, Merck, Mundipharma, Novartis, Novocure, Nuvation Bio, Prelude Therapeutics, Sapience Therapeutics, Servier, Tocagen, Vascular Biogenics and VBI Vaccines; and research funding from Agios, AstraZeneca, Bayer, Beigene, Celgene, Chimerix, Karyopharm Therapeutics, Kazia, MedicicNova, Merck, Novartis, Nuvation Bio, Oncoceutics, Puma Biotechnology, Vacular Biogenics and VBI Vaccines. M.W. has received research grants from Quercis and Versameb; and honoraria for lectures or advisory board participation or consulting from Bayer, Medac, Merck (EMD), Novartis, Orbus and Philogen. M.P. has received honoraria for lectures, consultation or advisory board participation from AbbVie, AstraZeneca, Bayer, BMJ Journals, Bristol Myers Squibb, CMC Contrast, Daiichi Sankyo, Gerson Lehrman Group, GlaxoSmithKline, Lilly, Medahead, MedMedia, Merck Sharp & Dohme, Mundipharma, Novartis, Roche, Sanofi and Tocagen; and institutional research support from AbbVie, Boehringer-Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, GlaxoSmithKline, Merck Sharp & Dohme, Novocure and Roche. R.B., J.C., H.K.G. and M.v.d.B. declare no competing interests. Peer review Peer review information Nature Reviews Clinical Oncology thanks P. Kumthekar, T. Johns and M. Valiente for their contribution to the peer review of this work. Additional information Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Related links
Denali Therapeutics Frequently Asked Questions (FAQ)
When was Denali Therapeutics founded?
Denali Therapeutics was founded in 2015.
Where is Denali Therapeutics's headquarters?
Denali Therapeutics's headquarters is located at 161 Oyster Point Blvd, South San Francisco.
What is Denali Therapeutics's latest funding round?
Denali Therapeutics's latest funding round is IPO.
How much did Denali Therapeutics raise?
Denali Therapeutics raised a total of $347M.
Who are the investors of Denali Therapeutics?
Investors of Denali Therapeutics include Baillie Gifford & Co., Alaska Permanent Fund, ARCH Venture Partners, Flagship Pioneering, F-Prime Capital and 3 more.
Who are Denali Therapeutics's competitors?
Competitors of Denali Therapeutics include Cava Healthcare and 8 more.
Compare Denali Therapeutics to Competitors

EnClear Therapies develops a treatment device to stop the progress of neurodegenerative diseases.

OptoCeutics develops medical light that can restore the neuronal activity of Alzheimer's patients and reduce plaque accumulation, which can lead to the development of new medical technologies. The technology is based on known gamma light therapy research, but combines the light in a new way that the flickering is non-perceptible and thus not hindering vision or causing discomfort. The firm was founded in 2018 and is based in Copenhagen, Denmark.
Cava Healthcare is focused on enhancing optimal health by predicting, preventing and alleviating diseases.

Altimmune (NASDAQ: ALT) offers immunotherapeutic solutions focused on the development of products to stimulate immune responses for the prevention and treatment of diseases. Its product pipeline includes peptide-based GLP-1/glucagon dual receptor agonists used for the treatment of obesity and non-alcoholic steatohepatitis and immunotherapeutics for chronic hepatitis B (CHB) infections. The company was founded in 1997 and is based in Gaithersburg, Maryland.
Roxro Pharma is an early-stage specialty pharmaceutical company dedicated to identifying, licensing, and developing compounds in specialized disease areas with significant sales potential, such as pain management. Roxro will pursue product development with the objective of commercializing these products. Niche products will not be developed by major pharmaceutical companies because they will not fuel their growth requirements. Foreign and biotechnology companies with compounds that have a limited sales potential will not be partnered with major pharmaceutical companies. Therefore there are a number of products not being developed even though they are efficacious, safe and satisfy an unmet medical need.
SYNAPSIN is developing therapeutic strategies for the treatmentand prevention ofneurodegenerative disorders such as Alzheimer's disease (AD),Parkinson's disease, Amyotrophic Lateral Sclerosis (ALS), and spinal cord injury.
Discover the right solution for your team
The CB Insights tech market intelligence platform analyzes millions of data points on vendors, products, partnerships, and patents to help your team find their next technology solution.