Homology Medicines
Founded Year
2015Stage
Corporate Minority - P2P | IPOTotal Raised
$127.07MMarket Cap
0.06BStock Price
0.98Missing: Homology Medicines's Product Demo & Case Studies
Promote your product offering to tech buyers.
Reach 1000s of buyers who use CB Insights to identify vendors, demo products, and make purchasing decisions.
Missing: Homology Medicines's Product & Differentiators
Don’t let your products get skipped. Buyers use our vendor rankings to shortlist companies and drive requests for proposals (RFPs).
Expert Collections containing Homology Medicines
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
Homology Medicines is included in 2 Expert Collections, including Biopharma Tech.
Biopharma Tech
15,535 items
Companies involved in the research, development, and commercialization of chemically- or biologically-derived therapeutic & theranostic drugs. Excludes vitamins/supplements, CROs/clinical trial services.
Regenerative Medicine
1,818 items
Regenerative medicine refers to the process of activating, replacing, engineering or regenerating human genetic material, cells, tissues or organs to restore normal function. It also includes bioengineered tissues used for in vitro testing (e.g. organ-on-a-chip, organoids).
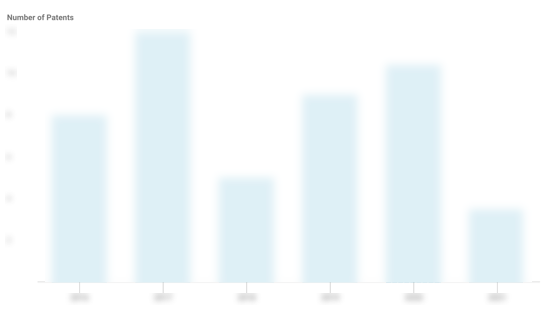
Homology Medicines Patents
Homology Medicines has filed 20 patents.
The 3 most popular patent topics include:
- Rare diseases
- Autosomal recessive disorders
- Syndromes
Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
2/19/2019 | 4/19/2022 | Molecular biology, Transcription factors, Genetics, Biotechnology, Rare diseases | Grant |
Application Date | 2/19/2019 |
|---|---|
Grant Date | 4/19/2022 |
Title | |
Related Topics | Molecular biology, Transcription factors, Genetics, Biotechnology, Rare diseases |
Status | Grant |
Latest Homology Medicines News
May 3, 2023
Targeted Approach to Immunosuppression with AAV Gene Therapy: Nonclinical Support of Clinical Approaches Thursday, May 18 at 12:00 p.m. PT Abstract # 989 Gene Therapy Candidate for Metachromatic Leukodystrophy (MLD): Optimization of HMI-202 Leading to HMI-204 Nomination Friday, May 19 at 12:00 p.m. PT Abstract # 1312 AAVHSC Re-Dosing Re-Dosing of Liver-Targeted AAV within and Across Clades in Mice: Effects of Neutralizing Antibodies and Vector-Specific Factors Friday, May 19 at 12:00 p.m. PT Abstract # 1355 AAVHSC-Mediated, Homologous Recombination-Based Gene Editing Method for Identification and Characterization of Sites of Homology Directed Strand Cross-Over Using rAAV Integration Vectors Friday, May 19 at 12:00 p.m. PT Abstract # 1497 The abstracts are available on the ASGCT website and on the Publications and Presentations page on Homology’s website. About Homology Medicines, Inc. Homology Medicines, Inc. is a clinical-stage genetic medicines company dedicated to transforming the lives of patients suffering from rare diseases by addressing the underlying cause of the disease. The Company’s clinical programs include HMI-103, a gene editing candidate for phenylketonuria (PKU); HMI-203, an investigational gene therapy for Hunter syndrome; and HMI-102, an investigational gene therapy for adults with PKU. Additional programs focus on paroxysmal nocturnal hemoglobinuria (PNH), metachromatic leukodystrophy (MLD) and other diseases. Homology’s proprietary platform is designed to utilize its family of 15 human hematopoietic stem cell-derived adeno-associated virus (AAVHSCs) vectors to precisely and efficiently deliver genetic medicines in vivo through a nuclease-free gene editing modality, gene therapy, or GTx-mAb, which is designed to produce antibodies throughout the body. Homology established an AAV manufacturing and innovation business in partnership with Oxford Biomedica, which was based on Homology’s internal process development and manufacturing platform. Homology has a management team with a successful track record of discovering, developing and commercializing therapeutics with a focus on rare diseases. Homology believes its initial clinical data and compelling preclinical data, scientific and product development expertise and broad intellectual property position the Company as a leader in genetic medicines. For more information, visit www.homologymedicines.com. Forward-Looking Statements This press release contains forward-looking statements. We intend such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including without limitation statements regarding: our expectations surrounding the potential, safety, efficacy, and regulatory and clinical progress of our product candidates, including HMI 104 for the treatment of PNH and other diseases; the potential of our gene therapy and gene editing platforms, including our GTx-mAb platform; our plans and timing for the release of additional preclinical and clinical data; our plans to progress our pipeline of genetic medicine candidates and the anticipated timing for these milestones; our position as a leader in the development of genetic medicines and our participation in upcoming presentations and conferences. The words “believe,” “may,” “will,” “estimate,” “potential,” “continue,” “anticipate,” “intend,” “expect,” “could,” “would,” “project,” “plan,” “target,” and similar expressions are intended to identify forward-looking statements, though not all forward-looking statements use these words or expressions. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: we have and expect to continue to incur significant losses; our need for additional funding, which may not be available; failure to identify additional product candidates and develop or commercialize marketable products; the early stage of our development efforts; potential unforeseen events during clinical trials could cause delays or other adverse consequences; risks relating to the regulatory approval process; interim, topline and preliminary data may change as more patient data become available, and are subject to audit and verification procedures that could result in material changes in the final data; our product candidates may cause serious adverse side effects; inability to maintain our collaborations, or the failure of these collaborations; our reliance on third parties, including for the manufacture of materials for our research programs, preclinical and clinical studies; failure to obtain U.S. or international marketing approval; ongoing regulatory obligations; effects of significant competition; unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives; product liability lawsuits; securities class action litigation; the impact of the COVID-19 pandemic and general economic conditions on our business and operations, including our preclinical studies and clinical trials; failure to attract, retain and motivate qualified personnel; the possibility of system failures or security breaches; risks relating to intellectual property; and significant costs incurred as a result of operating as a public company. These and other important factors discussed under the caption “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2022 and our other filings with the Securities and Exchange Commission could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management’s estimates as of the date of this press release. While we may elect to update such forward-looking statements at some point in the future, we disclaim any obligation to do so, even if subsequent events cause our views to change. Company Contacts:
Homology Medicines Frequently Asked Questions (FAQ)
When was Homology Medicines founded?
Homology Medicines was founded in 2015.
Where is Homology Medicines's headquarters?
Homology Medicines's headquarters is located at 1 Patriots Park, Bedford.
What is Homology Medicines's latest funding round?
Homology Medicines's latest funding round is Corporate Minority - P2P.
How much did Homology Medicines raise?
Homology Medicines raised a total of $127.07M.
Who are the investors of Homology Medicines?
Investors of Homology Medicines include Pfizer, Novartis, 5AM Ventures, Deerfield Management, ARCH Venture Partners and 11 more.
Discover the right solution for your team
The CB Insights tech market intelligence platform analyzes millions of data points on vendors, products, partnerships, and patents to help your team find their next technology solution.