Medable
Founded Year
2012Stage
Series D | AliveTotal Raised
$533.75MValuation
$0000Last Raised
$304M | 2 yrs agoMosaic Score The Mosaic Score is an algorithm that measures the overall financial health and market potential of private companies.
+10 points in the past 30 days
About Medable
Medable operates as a global platform for decentralized clinical trials. It solves the systemic challenges inherent in modern clinical trials including access, interoperability between systems, and inefficient technology experiences. It was formerly known as Dermatrap. It was founded in 2012 and is based in Palo Alto, California.
Compete with Medable?
Ensure that your company and products are accurately represented on our platform.
Medable's Products & Differentiators
Medable Platform
To create a seamless experience for site and patients, all of our products are integrated into a single platform. The core Platform contains portals for the site, patient, and sponsor in both web and mobile modalities.
Research containing Medable
Get data-driven expert analysis from the CB Insights Intelligence Unit.
CB Insights Intelligence Analysts have mentioned Medable in 10 CB Insights research briefs, most recently on Jul 18, 2022.
Expert Collections containing Medable
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
Medable is included in 9 Expert Collections, including Unicorns- Billion Dollar Startups.
Unicorns- Billion Dollar Startups
1,208 items
Regtech
1,341 items
Technology that addresses regulatory challenges and facilitates the delivery of compliance requirements in FIs. Regulatory technology helps FIs and regulators address challenges ranging from traditional compliance and risk management to data reporting and transmission.
Clinical Trials Tech
341 items
Companies developing products and services to streamline drug R&D, from drug discovery, pre-clinical testing, and clinical trials.
Conference Exhibitors
5,302 items
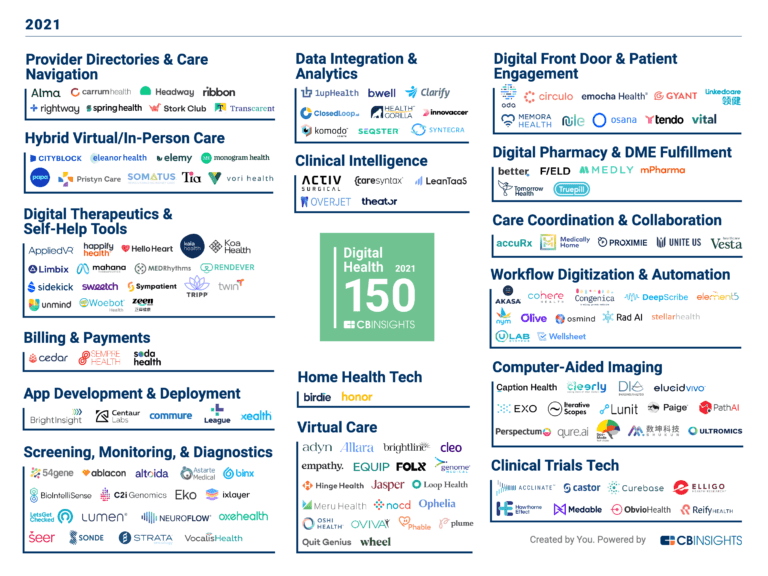
Digital Health 150
300 items
The winners of the second annual CB Insights Digital Health 150.
Digital Health
11,233 items
The digital health collection includes vendors developing software, platforms, sensor & robotic hardware, health data infrastructure, and tech-enabled services in healthcare. The list excludes pureplay pharma/biopharma companies and and assistive tech developers.
Medable Patents
Medable has filed 5 patents.
The 3 most popular patent topics include:
- Healthcare occupations
- Classification algorithms
- Computer security
Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
5/24/2017 | 4/18/2023 | Graphical user interface elements, Health informatics, Medical ethics, Graphical control elements, Graphical user interfaces | Grant |
Application Date | 5/24/2017 |
|---|---|
Grant Date | 4/18/2023 |
Title | |
Related Topics | Graphical user interface elements, Health informatics, Medical ethics, Graphical control elements, Graphical user interfaces |
Status | Grant |
Latest Medable News
Apr 25, 2023
Global Cross-Industry Collaboration Continues Sharing Outputs to Improve Research Access SAN DIEGO, April 25, 2023 (GLOBE NEWSWIRE) -- The Decentralized Trials & Research Alliance (DTRA) today has announced the release of Patient Journey Maps to help researchers understand participation considerations and burden and to maximize patient inclusion, diversity, and participation in research. The Team comprised leaders in patient experience in clinical research who chose three areas for initial focus: Oncology, Rare Disease, and Vaccines. These journeys are the result of conversations with patients along with lessons learned from industry leaders implementing decentralized research methods. “The patient journey maps can be used as guidelines for others in the industry to find ways to truly embrace the patient perspective when it comes to decentralized clinical trials,” said Alicia Staley, Vice President of Patient Engagement at Medidata and Co-Lead of the DTRA Patient Journey Map initiative. "This was an amazing opportunity to collaborate with diverse partners throughout the clinical research industry, and gain a wide range of perspectives and ideas. Our team delivered a unique set of patient journey maps across three different therapeutic areas, which will enhance access and engagement in clinical trials.” The Patient Journey Maps as well as a blank template to be used for any therapeutic area are now available to the global research community at www.dtra.org . By visualizing the decision points that patients face in their treatment and clinical research experience, study teams can fully appreciate and anticipate the patient journey. With this information, researchers can then select the right designs and tools to support participation. The maps help demystify what methods to consider, and how that may differ across patient demographics and disease states. Using Patient Journey Maps ultimately helps trial teams with their decision making by providing examples to catalyze their thinking. The initiative team also created a template for teams to begin with a patient journey for their own study design and decision making. Together these tools support the idea of patient-centered design, and the need to consider patient experience as part of trial design and conduct. "Even though each patient is an ‘n of one’, the DTRA Patient Journey Map initiative was an opportunity to thoughtfully consider the unmet needs, quality of life impacts, and preferences of those mulling over clinical trial participation,” shared Richie Kahn, former Senior Director of Patient Success at Medable and current Co-Founder & Principal at Canary Advisors and Co Lead of the initiative. "By better aligning clinical trial protocols with patient wants and needs, we can reduce the time it takes to bring promising, new therapies to the patients that need them most." “We have successfully identified the patient journey for incorporating Decentralized Clinical Trials (DCTs) in clinical trials. This groundbreaking project utilizes a collaborative approach, incorporating diverse perspectives from multiple stakeholders, including patients, to optimize three distinct therapeutic trial types,” said Deena Bernstein, Vice President of Customer Success at DataCubed Health and Project Manager for the DTRA Patient Journey Map initiative. Having reached this milestone, the comprehensive maps serve as a valuable resource for all stakeholders when considering the implementation of tools to improve research access including decentralized methods in clinical trial designs. Accessible and free to all on the DTRA website, this user-friendly tool empowers users to customize the maps for their own unique needs, paving the way for a more thoughtful and intentional approach to clinical trials. ABOUT DTRA: The Decentralized Trials & Research Alliance ( DTRA ), a non-profit organization, was convened to enable collaboration of stakeholders to accelerate the adoption of patient-focused, decentralized clinical trials and research within life sciences and healthcare through education and research. It works to make research participation accessible to everyone, enabled by the consistent, widespread adoption of appropriate decentralized research methods. Follow DTRA on Twitter and LinkedIn for more information. To get more information about becoming an Organizational or Individual Member of the DTRA, visit www.dtra.org/join-today For further information please contact: Media Contact:
Medable Frequently Asked Questions (FAQ)
When was Medable founded?
Medable was founded in 2012.
Where is Medable's headquarters?
Medable's headquarters is located at 525 University Avenue, Palo Alto.
What is Medable's latest funding round?
Medable's latest funding round is Series D.
How much did Medable raise?
Medable raised a total of $533.75M.
Who are the investors of Medable?
Investors of Medable include GSR Ventures, Sapphire Ventures, Western Technology Investment, Tiger Global Management, Blackstone and 10 more.
Who are Medable's competitors?
Competitors of Medable include Alira Health, JNPMEDI, Medocity, Jeeva, WKD.SMRT, Delve Health, Curebase, Huma, Clara Health, Lindus Health and 21 more.
What products does Medable offer?
Medable's products include Medable Platform and 2 more.
Who are Medable's customers?
Customers of Medable include GSK and Syneos.
Compare Medable to Competitors

Curebase provides decentralized clinical research software solutions. It reduces recruitment times, automates manual steps, and lets drug companies distribute their trials to clinics. It was founded in 2017 and is based in San Francisco, California.
THREAD is a virtual research platform used by biopharma, CROs, non-profit researchers, and life science organizations to capture global clinical study data in between, as well as during and instead of clinic visits.

Reify Health provides cloud-based software that accelerates the development of new and life-saving therapies. Reify Health aims to change how clinical trials are run through its StudyTeam and Care Access platforms. StudyTeam delivers a technology platform for optimizing patient recruitment and enrollment. Care Access, which conducts decentralized trials at scale, is a decentralized research organization that aims to bring clinical trial infrastructure directly to patients, healthcare providers, and communities. Reify Health was founded in 2012 and is based in Boston, Massachusetts.
Castor EDC specializes in electronic data capture for medical research. It aims to take the clinical trial process which it describes as largely being offline and paper-based and digitizes it via software.

Evidation operates as a multichannel health tracking platform. It enables users to connect with multiple appications to track everyday activity and earn rewards for steps, sleep, surveys, and more. The company was founded in 2012 and is based in San Mateo, California.

Saama Technologies is a clinical data and analytics company. Saama's unified, AI-driven clinical data analytics platform integrates, curates, and animates unlimited sources of structured, unstructured, and real-world data to deliver insights across all therapeutic areas. The platform gives real-time visibility into clinical data, enabling sponsors to file New Drug Applications (NDAs) more efficiently to bring drugs to market faster and at lower costs. Saama Technologies was founded in 1997 and is based in Campbell, California.
Discover the right solution for your team
The CB Insights tech market intelligence platform analyzes millions of data points on vendors, products, partnerships, and patents to help your team find their next technology solution.