Mindstrong
Founded Year
2014Stage
Dead | DeadTotal Raised
$160.25MAbout Mindstrong
Mindstrong ceased operations in March 2023. It provides a digital therapeutic platform to address personal, societal, and economic problems. The platform offers a biomarker panel that measures brain function from interaction patterns captured passively and continuously from human-computer interfaces. The company was founded in 2014 and is based in Mountain View, California.
Missing: Mindstrong's Product Demo & Case Studies
Promote your product offering to tech buyers.
Reach 1000s of buyers who use CB Insights to identify vendors, demo products, and make purchasing decisions.
Missing: Mindstrong's Product & Differentiators
Don’t let your products get skipped. Buyers use our vendor rankings to shortlist companies and drive requests for proposals (RFPs).
Research containing Mindstrong
Get data-driven expert analysis from the CB Insights Intelligence Unit.
CB Insights Intelligence Analysts have mentioned Mindstrong in 9 CB Insights research briefs, most recently on Apr 24, 2023.

Apr 18, 2023
452 startup failure post-mortems
Feb 16, 2022
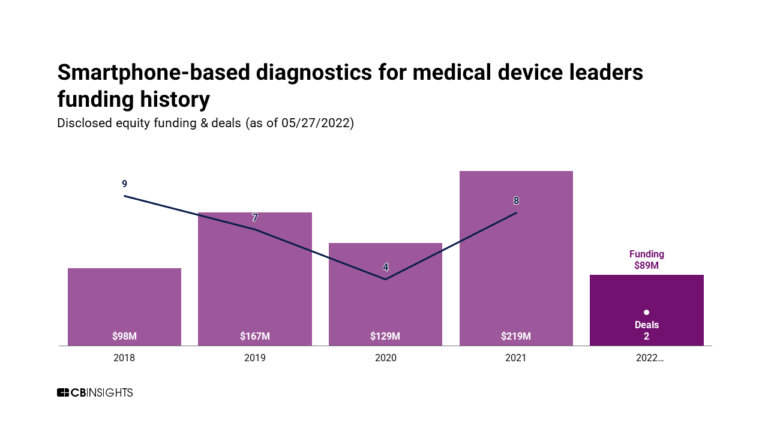
The 130+ AI startups reinventing healthcareExpert Collections containing Mindstrong
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
Mindstrong is included in 13 Expert Collections, including Mental Health Tech.
Mental Health Tech
1,285 items
This collection includes companies applying technology to problems of emotional, psychological, and social well-being. Examples include companies working in areas such as substance abuse, eating disorders, stress reduction, depression, PTSD, and anxiety.
AI 100
100 items
Artificial Intelligence
10,624 items
This collection includes startups selling AI SaaS, using AI algorithms to develop their core products, and those developing hardware to support AI workloads.
Medical Devices
8,875 items
Companies developing medical devices (per the IMDRF's definition of "medical device"). Includes software, lab-developed tests (LDTs), and combination products. *Columns updated as regularly as possible.
Health Monitoring & Diagnostics
2,768 items
Companies developing or offering products that aid in the assessment, screening, diagnosis, or monitoring of a person's state of health/wellness. Excludes companies focused solely on fitness/sports performance
Digital Health 150
150 items
The winners of the second annual CB Insights Digital Health 150.
Mindstrong Patents
Mindstrong has filed 9 patents.
The 3 most popular patent topics include:
- Cognitive tests
- Neuropsychological tests
- Cognition
Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
10/21/2016 | 7/4/2017 | Dietary supplements, Neuropsychology, Email, Nutrition, Cognition | Grant |
Application Date | 10/21/2016 |
|---|---|
Grant Date | 7/4/2017 |
Title | |
Related Topics | Dietary supplements, Neuropsychology, Email, Nutrition, Cognition |
Status | Grant |
Latest Mindstrong News
Apr 18, 2023
Adobe Over the past five years, digital mental health has risen from a niche topic to a global health priority. Patients, researchers, regulators, and investors alike are thrilled by the potential of ubiquitous mobile technology like smartphones to help diagnose problems, monitor health, and even deliver evidence-based therapies. Two companies that came to embody this potential were Mindstrong for smartphone monitoring of mental health and Pear Therapeutics for FDA-approved apps and digital interventions. Mindstrong raised nearly $160 million, and Pear Therapeutics once had a $1.6 billion evaluation. But in March, Mindstrong announced it was ceasing operations . And on April 7, Pear Therapeutics announced that it was filing for bankruptcy. Both companies were founded by smart and passionate people who wanted to improve access to mental health care. They were staffed by hard-working employees who wanted to help change the world. The concept of using data from personal patterns of smartphone use to help diagnose a mental illness or track recovery is appealing. So is the concept of using a smartphone app to receive evidence-based care from the palm of your hand. Pear Therapeutics even received some of the first Food and Drug Administration clearances for software as a medical device for mental health. advertisement But the companies went wrong in a way that is symptomatic of the rest of the digital mental health field. Both companies tackled the pressing need for a transformation in mental health care. They drew on the global need for a paradigm shift in mental health and offered a scientific pathway toward that vision. Mindstrong built off a 2018 pilot study of 27 subjects capturing smartphone data over seven days to pilot digital biomarkers of cognitive function, which could remotely diagnose or monitor mental health conditions. Pear Therapeutics had many products but was likely most well-known for its apps that promised to help treat addiction. advertisement The story of using technology to monitor and help support mental health made sense five years ago and in fact still makes sense today. Digital technology will help increase access to and quality of mental health care. But the story only goes so far. The next step is to prove out the story with science. Just as a new drug for a mental illness like depression undergoes numerous careful studies on its pathway to approval, the same process is necessary for digital software. Both Mindstrong and Pear Therapeutics undertook research and began the scientific journey. In Mindstrong’s case, the company founders have come out on the record stating they felt pressured to commercialize too soon and before the science was sound. Looking in the scientific literature, the lack of peer-reviewed publications corroborates this narrative. We need more research before smartphone sensing to detect mental illness is even clinically validated, let alone commercially viable for a business like Mindstrong. Pear Therapeutics appeared to have the evidence since it obtained FDA clearance on some of its software. Yet in 2020 the Institute for Clinical and Economic Review wrote a public report noting that the “evidence is inadequate to demonstrate a net health benefit.” In fact, that evidence was not based on any smartphone at all. It came from a related computer program called the Therapeutic Education System. This was tested in 2014, in a study where patients came twice a week, in person, to a clinic to log on and use the program on computers in the clinic. Even in 2014, the authors of that study wrote “additional research is needed to assess effectiveness in non-specialty clinical settings.” Pear Therapeutics acquired the rights to this program, worked to get an adapted version FDA-cleared, and then marketed it as a digital therapeutic. (It’s worth noting that there is no consensus on the definition of a digital therapeutic .) Nearly a decade later, it’s still unclear whether this system can work outside of a specialty clinic. Thus, in both the case of Mindstrong and Pear Therapeutics, there was no dramatic story of something going wrong. The problem was that neither company was able to invest the money to carry out the necessary scientific research. While Mindstrong and Pear Therapeutics are two of the most well-known, the lack of resources for clinical science is widespread in the industry . A 2022 report suggested that 44% of digital health companies has a clinical robustness score of zero. But that does not mean these products and digital mental health have no future. These failures tell us that patients, clinicians, and payers want digital tools that are effective in real-world settings. That means promising pilot studies, like those that launched this industry in the 2010s, must be followed by high-quality studies. When my colleagues and I conducted a meta-review in 2022, we found that overall, the quality of most digital mental health studies was low. Now it is time to think about a new generation of evidence and new studies to build the type of real-world data we need to ensure products offered are both safe and truly effective. This means studies that account for digital placebo effects, are run in real-world clinical settings, involve diverse patients, and can be replicated by outside teams. We already know how to do this. Pharmaceutical companies offer one example of high investment in medical research and development. They invest in thousands of potential drugs and take the top-performing ones through more and more rigorous studies. The National Institute of Mental Health is another example, now investing heavily in digital research and supporting rigorous mechanism of action studies. As patients, payers, and investors demand more evidence and proof that digital software really works as promised, new companies will rise to the challenge. Of course, there is a major difference between drug R&D and digital mental health care, which is that software needs additional research to prove it can successfully work in the context it is deployed in. But even this is a surmountable hurdle. Digital mental health might be stumbling in 2023, but these disappointments may signal a new era in how we approach the next generation of digital tools. The stories that founded and sustained both MindStrong and Pear Therapeutics are still valid today — they are just waiting for the next generation to take them off the page and into a scientific reality. John Torous is an assistant professor of psychiatry at Harvard Medical School and staff psychiatrist at Beth Israel Deaconess Medical Center. He is the scientific advisor for Precision Mental Wellness, a company he holds equity in. About the Author Reprints
Mindstrong Frequently Asked Questions (FAQ)
When was Mindstrong founded?
Mindstrong was founded in 2014.
Where is Mindstrong's headquarters?
Mindstrong's headquarters is located at 303 Bryant St, Mountain View.
What is Mindstrong's latest funding round?
Mindstrong's latest funding round is Dead.
How much did Mindstrong raise?
Mindstrong raised a total of $160.25M.
Who are the investors of Mindstrong?
Investors of Mindstrong include Mission Daybreak, Optum Ventures, Foresite Capital, ARCH Venture Partners, General Catalyst and 8 more.
Who are Mindstrong's competitors?
Competitors of Mindstrong include Alto Neuroscience, uMore, Cumulus Neuroscience, Feel Therapeutics, Gemelli Biotech and 10 more.
Compare Mindstrong to Competitors

LifeQ is a multinational science-driven technology and analytics company that combines skills from various disciplines (biomedical engineering, theoretical and experimental physics, systems biology, life and behavioral sciences, hardware engineering, systems engineering, computer science, digital signal processing, machine learning and other big data analytics disciplines, mathematics and mathematical statistics, systems theory, etc.) to understand how physiology, anatomy, behavior, and the environment interact, as well as how human health and wellness can be improved with this understanding. LifeQ provides biometrics and health information derived from wearable devices.

Cogniant operates as a technology and automated patient engagement solution company. The company offers mental health, digital phenotyping, remote monitoring, telehealth, deep learning, and passive data tracking solutions. The company supports researchers, clinicians, and biopharma companies along the value chain of clinical research and drug discovery for psychiatric and neurodegenerative disorders. It was founded in 2019 and is based in Singapore, Singapore.

Ellipsis Health develops artificial intelligence (AI) generated vocal biomarker technology. It helps in identifying, assessing, and monitoring mental health with a short voice sample, its technology determines the severity of anxiety and depression. Ellipsis Health was founded in 2017 and is based in San Francisco, California.

Headspace develops an online membership platform offering guided meditation and mindfulness. The company operates a business-to-business (Headspace for Work) to offer its mindfulness products and services to sectors such as government entities, nongovernmental organizations, and National Health Service (NHS) to offer digital mental health tools. It was founded in 2010 and is based in Santa Monica, California.

Beacon Biosignals' EEG neurobiomarker platform is engineered to accelerate clinical trials and enable new treatments for patients with neurological and psychiatric disease.
Sanvello is a new class of on-demand mobile therapy solutions for those seeking relief from anxiety, depression, stress, and other behavioral health conditions. This nationwide behavioral health provider business leverages tele-health and digital engagement tools in new ways, with a strong focus on primary care integration. The company is dedicated to helping people build the life skills they need; anytime, anywhere, and in any way they choose.
Discover the right solution for your team
The CB Insights tech market intelligence platform analyzes millions of data points on vendors, products, partnerships, and patents to help your team find their next technology solution.